V. Badets, J. Pandard, N. Sojic, S. Arbault
ChemElectroChem, 2016, 3, 2288-2296.
The reactivity of platinized electrode surface (Pt‐black) towards the oxidation of hydrogen peroxide is analyzed in the most common physiological buffer, namely phosphate buffer saline, used in the development of biosensors. It is shown here that the oxidation of hydrogen peroxide by Pt‐black involves first the adsorption of phosphate anions, which facilitates the oxidation of hydrogen peroxide at lower potentials when compared to classic polycrystalline platinum. This gives insight in the excellent analytical features of platinized electrodes for the detection of H2O2, even at nanomolar concentrations. The reactivity of platinized electrodes can be further improved by an oxygen plasma treatment. We demonstrate herein that such treatment creates an outermost layer of PtO2 oxides on the deposit, which improves the oxidation of hydrogen peroxide thermodynamically and kinetically, as well as of hydroquinone and superoxide radical anion.
F. Girard, V. Badets, S. Blanc, K. Gazeli, L. Marlin, L. Authier, P. Svarnas, N. Sojic, F. Clément S. Arbault
RSC Advances, 2016, 6, 78457–78467.
Cold Atmospheric Plasmas (CAPs) or Non Thermal Plasmas (NTPs) are increasingly used for biomedical applications. Herein, we studied the interactions of such CAPs, typically atmospheric ionization waves produced in a helium–nitrogen mixture (He/1% N2) with a commonly used physiological liquid in biology, e.g. Phosphate Buffered Saline solution (PBS) at pH 7.4. Optical Emission Spectroscopy (OES) of the plasma phase revealed the formation in the He/1% N2 CAP of nitric oxide NO and hydroxyl HO˙ derivatives which can lead to numerous Reactive Oxygen and Nitrogen Species (RONS) after dissolution in the exposed PBS. Chemical changes in solution were first assessed by conductimetry and pHmetry; these experiments showed that an evaporation of the solution occurred under gas exposition and was amplified by the CAPs, being mostly related to the interaction between the ionization wave and the gas flow. Further, UV-visible absorption spectroscopy was used to identify and quantify long-lived RONS, namely nitrite (NO2−), nitrate (NO3−), as well as a short-lived species, i.e. peroxynitrite anion (ONOO−). The production in physiological solution of ONOO− under CAP exposure is demonstrated for the first time, based on experiments at two pH conditions (7.4 and 12) and on the analysis of decomposition kinetics of this unstable species. The combination of complementary physico-chemical techniques allows to decipher the complex reactivity of CAPs from the plasma phase to the liquid phase.
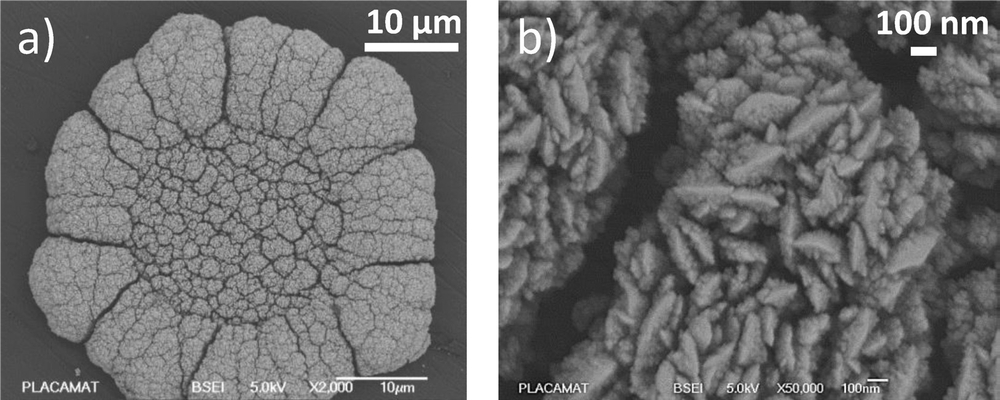
F. Sékli Belaïdi, E. Vanhove, W. Tiddi, M. Polverela, G. Lemercier, A. Lecestre, P. Dubreuil, J. Launay, S. Arbault, P. Temple-Boyer
Sensors Actuators B-Chemical, 2016, 232, 345-356.
In this work, we report the fabrication and the electrochemical characterization of recessed disk microelectrodes (DME) and ring nanoelectrodes (RNE) integrated in microwell arrays. Such configuration has all advantages of microelectrodes arrays but is more suitable for electrochemical measurement in sub-picolitre volumes (∼0.3 pL). The technological process based on the reactive ion etching of a SiO2/Ti/Pt/Ti/SiO2 stack is optimized in order to integrate RNE arrays on transparent glass substrate. Multiphysic simulations and electrochemical characterizations are conducted in order to study and improve the amperometric behaviour of recessed ring nanoelectrodes according to their geometry. A good fit is shown between experimental, theoretical and simulation results, allowing full understanding of the electrochemical detection properties of RNE-based microwell arrays. Then, a “generation − collection mode” chronoamperometric approach is proposed to evaluate experimentally the collection ratio of RNE arrays and compare it with simulation results. Finally, first electrochemical characterizations in sub-picolitre volumes are conducted with anti-oxidant species. All these results demonstrate that recessed ring nanoelectrode arrays are fitted to the detection of bio-electrochemical species at the microscale and, consequently to single mitochondrion or single sub-cellular organelle analysis.

V.S.R. Vajrala, E. Suraniti, M. Rigoulet, A. Devin, N. Sojic, S. Arbault
Integrative Biology, 2016, 8, 836-843.
Microwell arrays have been developed to monitor simultaneously, and on a large scale, multiple metabolic responses of single mitochondria. Wells of 50 to 1000 μm-diameter were prepared based on easy structuration of thin polydimethylsiloxane layers (PDMS; 100 μm thickness). Their surface treatment with oxygen plasma allowed the immobilization in situ and observation with time of populations of single isolated mitochondria. Their metabolic activities could be monitored individually by fluorescence microscopy under several activation/inhibition conditions. We measured the concomitant variations of two main metabolic parameters - the endogenous NADH level and the internal membrane potential difference Δψ owing to a cationic fluorescent probe (TMRM) - at energized, uncoupled and inhibited stages of the mitochondrial respiratory chain. Microwell arrays allowed analyses on large populations, and consequently statistical studies with a single organelle resolution. Thus, we observed rapid individual polarizations and depolarizations of mitochondria following their supply with the energetic substrate, while an averaged global polarization (increase of TMRM fluorescence within mitochondria) and NADH increase were detected for the whole population. In addition, statistical correlation studies show that the NADH content of all mitochondria tends toward a metabolic limit and that their polarization-depolarization ability is ubiquitous. These results demonstrate that PDMS microwell platforms provide an innovative approach to better characterize the individual metabolic status of isolated mitochondria, possibly as a function of their cell or organ origin or in different physio-pathological situations.

T. Yutthalekha,C. Wattanakit,V. Lapeyre,S. Nokbin,C. Warakulwit, J. Limtrakul, A. Kuhn
see also CNRS press release
The synthesis of chiral compounds is of crucial importance in many areas of society and science, including medicine, biology, chemistry, biotechnology and agriculture. Thus, there is a fundamental interest in developing new approaches for the selective production of enantiomers. Here we report the use of mesoporous metal structures with encoded geometric chiral information for inducing asymmetry in the electrochemical synthesis of mandelic acid as a model molecule. The chiral-encoded mesoporous metal, obtained by the electrochemical reduction of platinum salts in the presence of a liquid crystal phase and the chiral template molecule, perfectly retains the chiral information after removal of the template. Starting from a prochiral compound we demonstrate enantiomeric excess of the (R)-enantiomer when using (R)-imprinted electrodes and vice versa for the (S)-imprinted ones. Moreover, changing the amount of chiral cavities in the material allows tuning the enantioselectivity.
- Combining Microfluidics and FT-IR Spectroscopy: Towards spatially resolved information on chemical processes
- Antagonist Effects Leading to Turn-on Electrochemiluminescence in Thermoresponsive Hydrogel Films
- Selective Electrochemiluminescent Sensing of Saccharides using Boronic Acid-Modified Coreactant
- Essential Role of Electrode Materials in Electrochemiluminescence Applications






