Y. Fu, K. Chen, B. Xie, X. Zhang, L. Zhang, A. Kuhn, W. Yang
Chem. Mater. (2024) in press
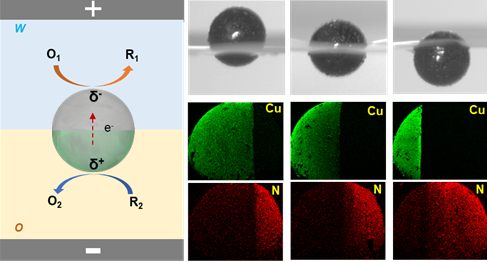
Janus particles have a wide range of applications in diverse fields due to the possibility to tailor and combine different functionalities on a single particle. However, most of the current techniques for the synthesis of Janus particles are still suffering from limited control of the generated asymmetry. Therefore, achieving the synthesis of bi-functionalized Janus particles with a completely tunable modification ratio between two components is still extremely challenging. In this context, bipolar electrochemistry (BE) offers unique advantages to achieve controlled asymmetry in terms of the precise spatial distribution of electrochemical reactions at the two polarized extremities of a bipolar electrode. We propose herein an approach to synthesize Janus particles at the water/organic (w/o) interface by bipolar electrochemistry. Janus particles with varying degrees of amphiphilicity are first prepared by bipolar electrochemistry, and are then positioned at the w/o interface. Subsequently, highly controlled bi-functionalization is achieved by carrying out different electrochemical reactions on their two sides. The ability to selectively modify the hydrophilic and hydrophobic regions allows for the generation of Janus particles with tailored properties at each face. This approach can be adapted for the synthesis of asymmetric particles with different dimensions, having various compositions and functionalities, thus opening up potential applications, ranging from catalysis and sensing to the delivery of active compounds.
R. Gao, M. Beladi-Mousavi, G. Salinas, L. Zhang, A. Kuhn
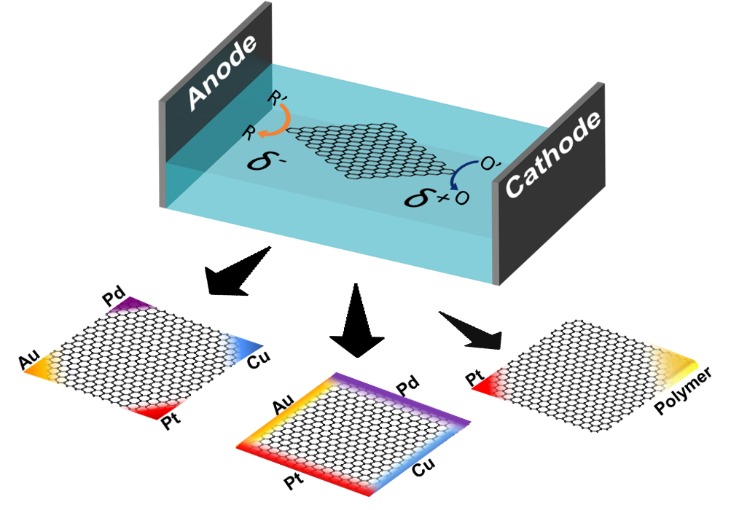
Graphene has gained substantial research interest in many fields due to its remarkable properties among many other two-dimensional materials. In this study, we propose a wireless electrochemical approach, bipolar electrochemistry, for the precise modification of single layers of graphene at predefined locations, such as distinct edges or corners, with a variety of metals or polymers, thus enabling the elaboration of multi-functional monolayer graphene sheets. We illustrate the concept e.g. by depositing multiple metals, or platinum and a catalyst-containing porous polymer on the same graphene sheet, but at separate corners. This configuration allows activating chemiluminescence on the polymer spot, and simultaneously generates the driving force for autonomous motion on the Pt side through the catalytic decomposition of hydrogen peroxide into oxygen bubbles. This integration of different chemical features on the same object, exemplified by these proof-of-principle experiments, enhances the functionality of two-dimensional materials, paving the way for the use of these hybrid materials for a variety of applications, ranging from sensing and catalysis to targeted delivery.
Yiran Zhao, Borja Sépulveda, Julie Descamps, Fatoumata Faye, Marcos Duque, Jaume Esteve, Lionel Santinacci, Neso Sojic, Gabriel Loget, Yoan Léger
ACS Appl. Mater. Interfaces 2024, 16, 9, 11722–11729
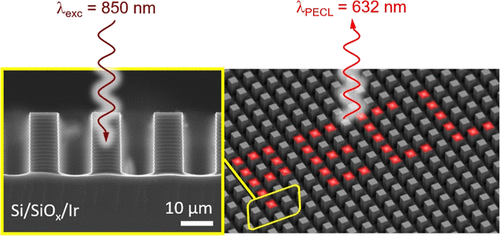
Infrared (IR) imaging devices that convert IR irradiation (invisible to the human eye) to a visible signal are based on solid-state components. Here, we introduce an alternative concept based on light-addressable electrochemistry (i.e., electrochemistry spatially confined under the action of a light stimulus) that involves the use of a liquid electrolyte. In this method, the projection of a near-IR image (λexc = 850 or 840 nm) onto a photoactive Si-based photoanode, immersed into a liquid phase, triggers locally the photoinduced electrochemiluminescence (PECL) of the efficient [Ru(bpy)3]2+-TPrA system. This leads to the local conversion of near-IR light to visible (λPECL = 632 nm) light. We demonstrate that compared to planar Si photoanodes, the use of a micropillar Si array leads to a large enhancement of local light generation and considerably improves the resolution of the PECL image by preventing photogenerated minority carriers from diffusing laterally. These results are important for the design of original light conversion devices and can lead to important applications in photothermal imaging and analytical chemistry.
J. Dabboussi, R.-A. Eichel, H. Kungl, R. Abdallah, G. Loget*
Curr. Opin. Electrochem., 2024, 45, 101468.
Despite the longstanding interest in urea oxidation reaction (UOR), the identification of reaction products under conventional conditions was only reported recently. It turns out that the initially thought “sustainable pathway”, leading to harmless products, represents just a small fraction of the overall reaction mechanism. This is detrimental as the use of urea-rich aqueous feeds for H2 production, along with their remediation through UOR, constitutes perhaps the most important added value of this process for power-to-X and clinical applications. Nonetheless, promising strategies favoring the formation of environmentally friendly products over harmful overoxidized ones already exist. This is expected to lead to a “rebirth” of this research field and open the quest for ultimate selectivity to ensure the complete sustainability of UOR. Therefore, the systematic analysis of reaction products, the elucidation of mechanisms for improving N2 faradaic efficiency, and the design of selective catalysts should be the next focus of research in the field of UOR.

Y. Zhao, Y. Léger, J. Descamps, N. Sojic, G. Loget
Electrochemiluminescence (ECL) is the generation of light induced by an electrochemical reaction, driven by electricity. Here, an all-optical ECL (AO–ECL) system is developped, which triggers ECL by the illumination of electrically autonomous “integrated” photoelectrochemical devices immersed in the electrolyte. Because these systems are made using small and cheap devices, they can be easily prepared and readily used by any laboratories. They are based on commercially available p-i-n Si photodiodes (≈1 € unit−1), coupled with well-established ECL-active and catalytic materials, directly coated onto the component leads by simple and fast wet processes. Here, a Pt coating (known for its high activity for reduction reactions) and carbon paint (known for its optimal ECL emission properties) are deposited at cathode and anode leads, respectively. In addition to its optimized light absorption properties, using the commercial p-i-n Si photodiode eliminates the need for a complicated manufacturing process. It is shown that the device can emit AO–ECL by illumination with polychromatic (simulated sunlight) or monochromatic (near IR) light sources to produce visible photons (425 nm) that can be easily observed by the naked eye or recorded with a smartphone camera. These low-cost off-grid AO-ECL devices open broad opportunities for remote photodetection and portable bioanalytical tools.

- Wireless Light-emitting Electrode Arrays for the Evaluation of Electrocatalytic Activity
- Fine-Tuning the optoelectronic and redox properties of an electropolymerized thiophene derivative for highly selective OECT-based zinc detection
- Infrared photoinduced electrochemiluminescence microscopy of single cells
- All-Optical Electrochemiluminescence at Metal-Insulator-Semiconductor Diodes






