I. Malytska, Th. Doneux, M. Bougouma, A. Kuhn, L. Bouffier.
J. Phys. Chem. C, 123, 2019, 5647
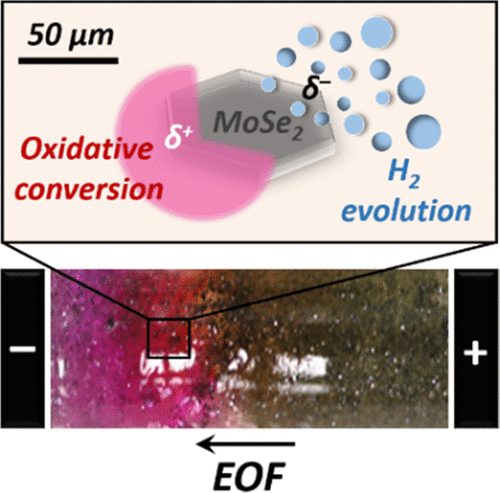
Doping of a transition-metal dichalcogenide deposited onto a conducting surface acting as bipolar electrode was recently reported. Here, freestanding macro- and microscale transition-metal dichalcogenide substrates are successfully employed as effective bipolar electrodes without the need of using an additional conducting support. This is first demonstrated by achieving site-selective bipolar electrodeposition of several metals, such as gold, silver, copper, and nickel, on macroscale MoSe2 substrates (typically 1 mm in size). Also, the superior efficiency of MoSe2 compared to that of a carbon substrate toward hydrogen evolution reaction, well-known in conventional electrochemistry, is demonstrated in the bipolar electrochemistry configuration. Such electrocatalytic properties can be advantageously used by combining this reduction with a given oxidation reaction to ease the electro-chemical coupling. Also, as a wireless technique, bipolar electrochemistry enables the simultaneous addressing of large ensembles of bipolar electrodes with a single pair of driving electrodes. Therefore, in a bulk experiment, a suspension composed of thousands of individual MoSe2 microparticles (with a typical size of 20−80 μm) that are addressed simultaneously, is employed to significantly accelerate electrolysis. Amplex Red was selected as an oxidizable organic model dye. Such electrolysis occurs on the timescale of several seconds, which is definitely not achievable by addressing a single macroscale MoSe2 bipolar electrode. This performance is due to the collective behavior of the ensemble of MoSe2 bipolar electrodes because the oxidation process occurs simultaneously at each individual anodic pole.