A. Perro, L. Giraud, N. Coudon, S. Shanmugathasan, B. Goudeau, J.-P. Douliez and V. Ravaine
Journal of Colloid and Interface Science 2019 548, 275-283.
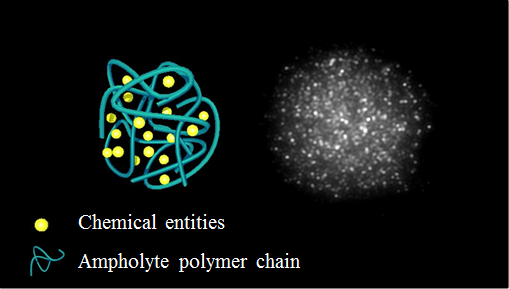
Coacervation is a phase separation process involving two aqueous phases, one solute-phase and one solute-poor phase. It is frequently observed among oppositely-charged polyelectrolyte systems. In this study, we focus on self-coacervation involving a single polymer chain and investigate its potential for encapsulation applications. Negatively charged polyacrylic acid polymer chains were partially cationized using diamine and carbodiimide chemistry affording ampholytes, named PAA-DA, with tunable charge ratio. When dispersed in water, at pH 7, PAA-DA was soluble but a phase separation occurs when decreasing pH close to the isoelectric point. Coacervation is found only for a given amine-to-acid ratio otherwise precipitation is observed. Increasing the pH above 4 yielded progressive destruction of the coacervates droplets via the formation of vacuoles within droplets and subsequent full homogeneous redispersion of PAA-DA in water. However, addition of calcium allowed increasing the coacervate droplet stability upon increasing the pH to 7 as the divalent ion induced gelation within droplets. Moreover, the coacervate droplets present the ability to spontaneously sequestrate a broad panel of entities, from small molecules to macromolecules or colloids, with different charges, size and hydrophobicity. Thanks to the reversible character of the coacervates, triggered-release could be easily achieved, either by varying the pH or by removing calcium ions in the case of calcium-stabilized coacervates. Self-coacervation presents the advantage of pathway-independent preparation, offering a real output interest in pharmacy, water treatment, food science or diagnostics.