Neso Sojic, Stéphane Arbault, Dodzi Zigah, Valérie Ravaine, Alexander Kuhn, Laurent Bouffier, Bertrand Goudeau, Patrick Garrigue
This thematic is focused on the development of new tools and methods for bioanalytical chemistry and nano-imaging by combining optics, luminescence and electrochemistry. Our activities in this field are organized as follow:
1. Optical Tools for Imaging & Enhanced Coupled Methodologies
2. Electrogenerated Chemiluminescence Imaging
3. Electrochemical Imaging
1. Optical Tools for Imaging & Enhanced Coupled Methodologies
High density nanoprobe arrays for DNA detection and for SERS nano-imaging are fabricated by wet etching optical fiber bundles. The resulting optical nanostructures keep the initial architecture of the bundle and therefore its intrinsic imaging properties. Remote SERS imaging was demonstrated through the bundle itself in collaboration within the ISM (group of molecular spectroscopy). We also developed with an industrial partner a remote imaging technique of living corneocytes directly on the human skin (L’Oréal).
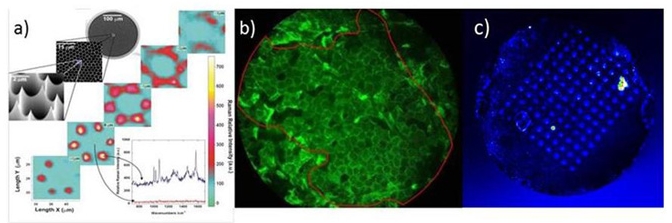
a) SERS with etched nanotip. b) Remote imaging of corneocytes on the forearm. c) Fluorescnece image of the DNA spots electropolymerized on the nanotip array.
Understanding mitochondrial metabolic status at the single organelle level is of major interest for several bio-medical fields, since there is a genetic and metabolic heterogeneity within the mitochondrial pool-network of each cell. In this context, we developed microwell arrays for fluorescence microscopy of single isolated mitochondria. We are working on the multiparametric monitoring of mitochondrial metabolic state using fluorescent dyes or endogenous fluorescent metabolites. Our goal is focused on the understanding of the kinetic relationships between mitochondrial membrane potential fluctuations and reactive oxygen and nitrogen species formation. We combine also such fluorescence microscopy approaches with miniaturized electrochemical sensors (see also the webpage Electrochemistry of Biosystems).
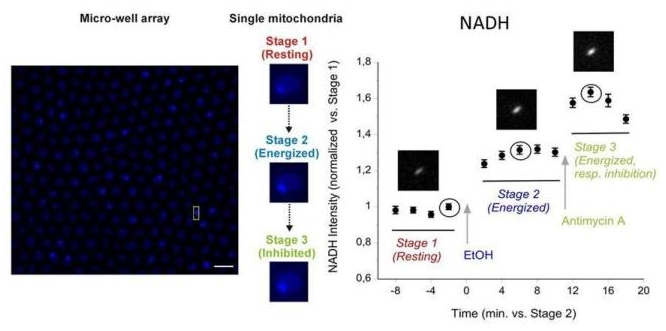 Monitoring of NADH fluorescence variations in individual mitochondria (from Saccharomyces cerevisiae) under different stages
Monitoring of NADH fluorescence variations in individual mitochondria (from Saccharomyces cerevisiae) under different stages
Stage 1: resting state of mitochondria; Stage 2: mitochondria energized by injecting ethanol 1% in the well; Stage 3: mitochondrial respiration inibited by injecting Antimycin A, a usual inhibitor of complex III in the respiratory chain.
2. Electrogenerated Chemiluminescence Imaging
Electrogenerated ChemiLuminescence (ECL) is the luminescence emitted by a luminophore resulting from an initial electron transfer reaction occurring at the electrode surface. In other words, it combines intimately electrochemistry and photochemistry. Immunoassays based on ECL are widely commercialized. We developed a novel photopatterning method for immobilisation of ECL ultrathin films. ECL hydrogel films were immobilised in form of uniform photopatterns which size, shape and thickness were modulated depending on the fabrication parameters. The design and implementation of a new class of sensing microarrays were also developed. ECL was used as a readout mechanism to detect multiple antigens simultaneously. The method enables multiplexed assays because all the individual sensing beads in the array are simultaneously imaged by ECL.
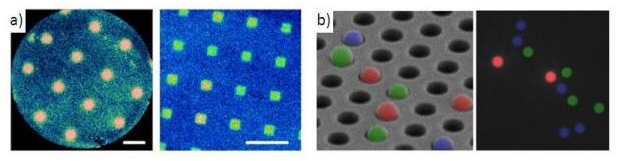 a) ECL images of different patterns of nanometer-thin films obtained on gold electrode. b) Platform for multiplexed sandwich immunoassays: antigens detection with ECL bead-based microarray.
a) ECL images of different patterns of nanometer-thin films obtained on gold electrode. b) Platform for multiplexed sandwich immunoassays: antigens detection with ECL bead-based microarray.
Particularly fascinating nanomaterials are stimuli-responsive hydrogel particles, or microgels. The properties of such so-called “smart” microgels are modulated by an external stimulus, which triggers expansion or contraction of the polymer network, at the origin of sensing capabilities (see the webpage Chemical Sensors for Biology). Through a rational choice of model ECL and microgel systems, we studied electrochemistry and ECL of thermo-responsive microgels. We demonstrated an unexpected enhancement of the ECL signal which occurs at the swell-collapse transition of the microgel particles.
Carbon nanotubes (CNTs) have emerged recently as a powerful analytical tool with a wide range of electronic and mechanic properties. For instance, in the context of electrochemical biosensors, CNTs are used to structure electrodes and increase the corresponding active surface. We are now developing an original approach based on tubes which are selectively modified on one side by bipolar electrochemistry, in order to generated unsymmetrical systems with promising analytical potential and improved detection capability. In order to locally modify this carbon tube an original approach using bipolar electrochemistry and diazonium salts reduction is used. The electroreduction of diazionium salts allow us to immobilize an organic layer using a covalent bonding. This organic layer can be used to develop analytical system.
 a) Schematic representation of the thermo-responsvie ECL microgels in the swollen (left) and collapsed (right) states; b) Transmission electron microscopy image of dried microgels; c) Asymmetric light-emitting swimmer. The synergetic reduction of H2O at the cathodic pole and oxidation of the ECL reagents at the anodic pole induces simultaneous motion and light emission of the bead in a capillary.
a) Schematic representation of the thermo-responsvie ECL microgels in the swollen (left) and collapsed (right) states; b) Transmission electron microscopy image of dried microgels; c) Asymmetric light-emitting swimmer. The synergetic reduction of H2O at the cathodic pole and oxidation of the ECL reagents at the anodic pole induces simultaneous motion and light emission of the bead in a capillary.
Bipolar electrochemistry, that usually has been employed in our group to modify objects in an asymmetric way (see paragraph on Janus particles on the webpage Smart and Dynamic Particles) can also be applied to the simultaneous bubble production and ECL generation on both poles of a conducting bead. It leads to the first example of a propulsion mechanism for a swimmer that is coupled with a chemical light source. In this case ECL provides a direct monitoring of the motion, which could be very useful for localizing micromotors. Changing the chemistry occurring at the surface of the moving object allows tuning the emitted colour, for example, using the classic luminol/H2O2 reaction leads to a blue light emitting swimmer.
Scanning Electrochemical Microscopy (SECM) has been first described in the early 90’s and is nowadays widely used to study interfacial phenomenon. By scanning an ultramicroelectrode (UME) above a surface, a three-dimensional image can be obtained. SECM can be used in order to obtain a topographic or/and chemical imaging, for example it is possible to observe the location of enzyme sites in a membrane, or to identify areas with different reactivity on a surface. For example, we studied a polypyrrole layer deposited on gold electrode by bipolar electrochemistry. Thanks to the SECM we proved that this layer presented various reactivity depending of its deposition potential.

a) Ultramicroelectrode and its diffusion layer close to the substrate; b) Substrate: polypyrrole on gold; c) SECM image, the high current (in red) corresponding to the gold layer.






